
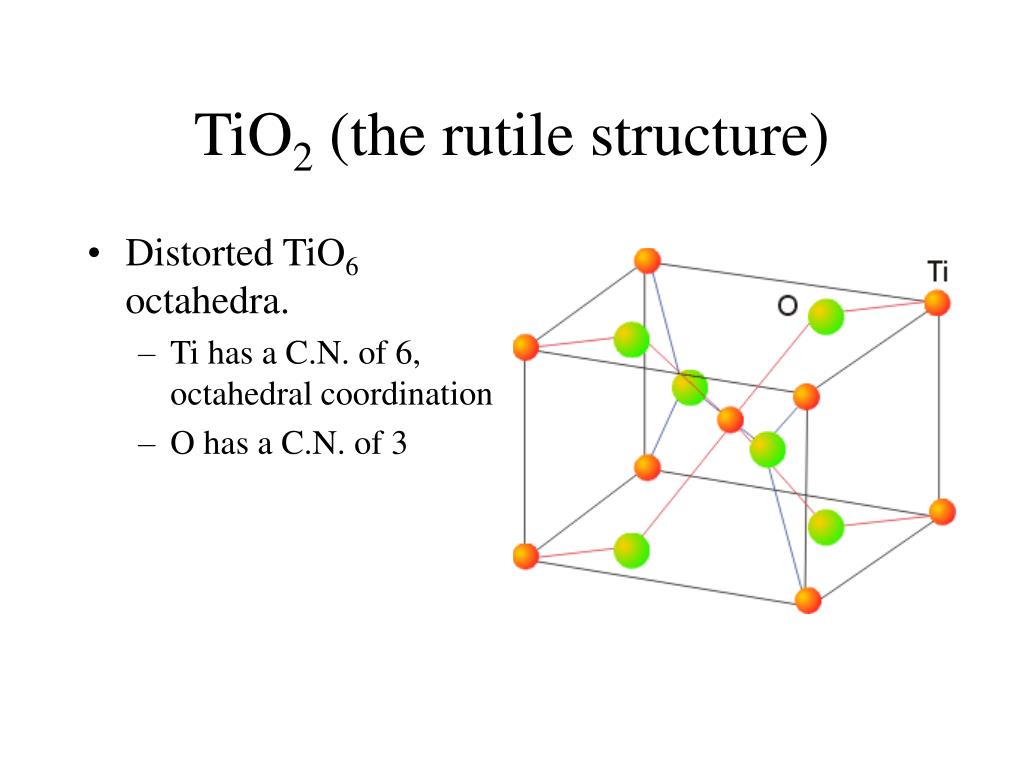
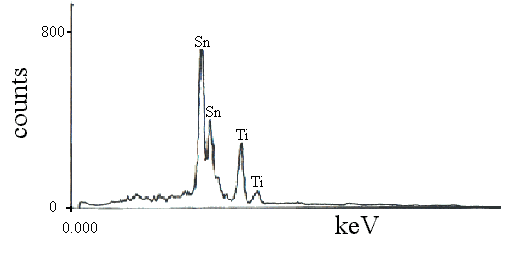
In the MO 6 coordination polyhedra, the M-O distances in the xy plane are 1.9080 (12) Å, while the M-O distances along the z axis increase to 1.9441 (19) Å. Relevant bond lengths and angles are presented in Table 1 ▸. The structure is represented by chains of edge-sharing MO 6 octahedra running parallel to the c-axis direction (Fig. 2 ▸) and connected to each other by shared corners. Each oxygen atom occupies a 4f position and is surrounded by three M sites, forming triangular MO 3 groups in the (110) lattice plane (Fig. 1 ▸). 57% of the 2a positions are occupied by Ge and the remaining 43% are occupied by Ti. The shared metal site M is in Wyckoff position 2a and is surrounded by six O atoms, thus forming a sixfold coordination polyhedron. The crystal structure of Ge 0.57Ti 0.43O 2 corresponds to the TiO 2 rutile type (space group P4 2/ mnm). At temperatures above 1873 K, crystal growth was significant and high-quality single crystals of the solid solution with a composition near TiGeO 4 could be obtained. Instead of forming Ge-bearing TiO 2 and Ti-bearing GeO 2, we discovered that the high pressure and temperature conditions led to the formation of a crystalline, single solid-solution material. We synthesized the title compound while investigating the GeO 2–TiO 2 phase diagram at a pressure of 8 GPa at 2028 K by means of the multi-anvil high-pressure technique.
TiO 2 rutile undergoes two phase transitions under high pressure of up to 12 GPa: rutile-to-α-PbO 2-type at around 7 GPa and α-PbO 2-to-baddeleyite at 12 GPa (Gerward & Staun Olsen, 1997 ▸). At pressures above 25 GPa, the tetragonal rutile-type phase transforms into an orthorhombic CaCl 2-type phase (Haines et al., 2000 ▸). GeO 2 is dimorphous at ambient atmospheric conditions, represented by both rutile-type and α-quartz-structured phases depending on the temperature, but with increasing pressure the GeO 2 rutile becomes more stable, and is the primary phase above two GPa (Micoulaut et al., 2006 ▸). The GeO 2–TiO 2 phase diagram at elevated pressures and temperatures has not been studied in great detail and the mutual solubility of Ge and Ti in the phases stable at these conditions is still largely unknown. Additionally, at ambient pressure GeO 2 and TiO 2 exhibit only limited mutual solubility. A metastable α-quartz-type structured GeO 2 has also been reported as the result of the cooling of the β-quartz-type structure (Sarver, 1961 ▸). At ambient pressure, the GeO 2–TiO 2 phase diagram shows the formation of three phases: rutile-type GeO 2, stable up to 1323 K, β-quartz-type GeO 2, stable above 1323 K and TiO 2 in the form of rutile.


 0 kommentar(er)
0 kommentar(er)
